Solid Solution Strengthening
High purity metals, while useful, are often softer and weaker than their alloys. The mechanical properties such as yield strength and tensile strength can be determined by the type and amount of impurity added.
One of the most common techniques to harden metals is Solid Solution Strengthening.
The process involves dissolving one metal into another (in a liquid state), this is known as casting.
The casting process involves pouring the liquid metal or metals into a mould and then leaving it to cool, the mould can be various shapes depending on the application. The base metal is known as the solvent and the impurity added is the solute.
There is a limit to the amount of solute that can be dissolved into the solvent, known as the solubility limit.
We are going to analyse the addition of Nickel to Copper
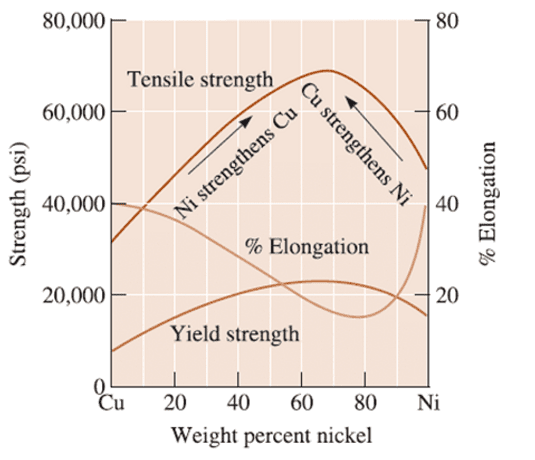
Figure 1 – effect of alloying Nickel with Copper
Figure 1 shows how the yield and tensile strength change with increasing amounts of Nickel added to copper. These charts can be used to determine the percentage necessary for the required characteristics. This is also important as the Nickel is more expensive than copper, so not adding excess is important.
Why do the Tensile strength and Yield change?
To explain these changes, we need to examine the structures at the atomic level.
- The impurity is surrounded by the host atoms imposing strains on the atoms within the material crystalline lattice.
- The strength is now increased due to dislocation movements becoming difficult within the new solid solution.
- Smaller impurities: exerts tensile strains on the surrounding crystal lattice.
- Larger impurities: compressive strains are imposed on the lattice.
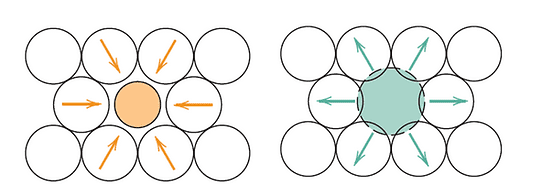
There are two types of solid solution strengthening, depending on the size of the impurity atoms, these are a substitutional solid solution (larger impurities) or an interstitial solid solution (smaller impurities)
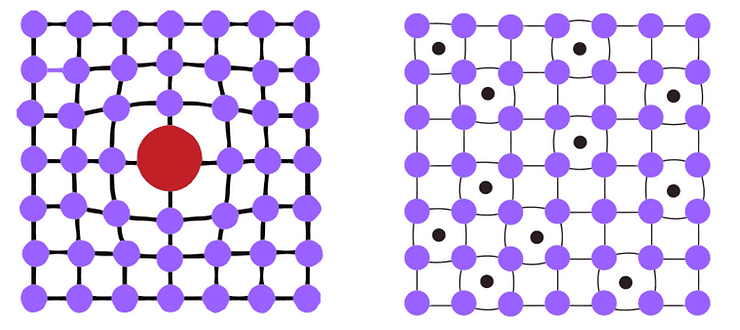
Figure 2a and 2b – Substitutional and interstitial solid solutions
Substitutional solid solutions
- The host atom is substituted with the alloying atom which imposes compressive strains on the neighbouring atoms.
- Due to the irregularity of the crystal lattice, dislocations cannot easily move around this interruption.
- It will take a much higher stress level or temperature to enable the dislocation to move again.
- Mainly volumetric changes
Interstitial solid solutions
- The host atoms are joined by smaller impurities, tensile strains are exerted on the surrounding crystal lattice structure.
- These interactions also prevent dislocations moving throughout the material.
- The added impurities exist in areas around the host atoms and move in between these atoms.
- Produce either volumetric or distortional strains.
Interstitial atoms are the most efficient strengthening method.
The strength of the solid is determined by the shape of the strain fields caused by the defects and dislocations.
Substitutional solutes generally stretch the lattice uniformly producing hydrostatic strain fields around the solutes. These hydrostatic strain fields are easy for further dislocation motion to overcome, so the strengthening effect is much less than interstitial solutes.
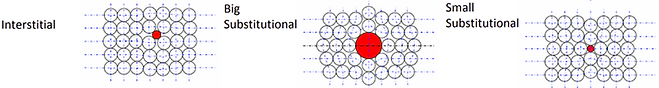
Conditions for strength of final solid solutions
There are four factors which can affect the strength of the final solid solution, they are as follows:
- Atomic Size: In order for the impurity atom to replace the host atom, the size of the atoms must be similar.
- Crystal Structure: The crystallization of the two atoms must be the same for the substitution to be successful.
- Electronegativity: The electronegativity needs to be the similar to prevent ionisation. Electronegativity is a measure of an atom’s ability to attract electrons.
- Valence: The valance must be the same (or similar) for the formation of the structure. Valence is a measure of an atom’s ability to combine with other atoms.
Conclusion
Solution hardening, or alloying, is a powerful method to improve the strength of a material. However, there are many other techniques we have not covered here.
Alloying does not only affect strength, but it can also improve other important properties, such as conductivity, elastic modulus, ductility, and cost.
All properties of a material must be scrutinized by the designer before a material choice can be made.
Interested in materials and their properties? You can study online, and acheive a Diploma in Material Science with iLean Engineering. Alternatively, view our full list of available courses here.
Why not check out the online engineering short courses specifically in manufacturing engineering:
Recent Posts
Aircraft Basics: Main Components and Standard Control Surfaces Explained
Aircraft Basics: Main Components and Standard Control Surfaces Explained Introduction In this blog we will identify the main components within an aircraft, more from the point of view of large external parts, more specifically, flight control surfaces. Flight control surfaces are simply physical devices that the pilot can control and adjust in order to change […]
Understanding and Calculating Generator Efficiency and Output Parameters
Understanding and Calculating Generator Efficiency and Output Parameters Introduction The performance of a generator is often judged by how efficiently it converts mechanical energy into electrical energy. Understanding and calculating this efficiency, along with other key output parameters such as voltage, current, power factor, and load, is essential for evaluating performance and ensuring reliable operation. […]
Essential Cooling and Protection Devices: How They Work and Why They Matter
Essential Cooling and Protection Devices: How They Work and Why They Matter Introduction Generators produce a significant amount of heat and electrical stress during operation, which can affect performance and lifespan if not properly managed. That’s where cooling and protection devices come in. These essential systems, including fans, radiators, circuit breakers, and relays, work together […]

