Engineering materials and their atomic structure
In our last article, we looked at how we can apply Bernoulli’s principle to engineering problems. Now we’re going to dive into engineering materials and their atomic structure.
Engineering Materials
Engineering can be defined as the application of science and technology to create or produce something. In order to produce something, we often mean processing or forming some sort of material into a useful shape or combination of shapes.
In order to do this, we need to understand what materials are, as well as how they can be processed and shaped. Materials can be broken down into 4 distinct groups:
- Metals
- Polymers
- Ceramics
- Composites
We need to look at the fundamental building block of all of these materials – atoms.
Structure of an atom
All substances around us, from our body to the Earth and sky, are made up of atoms. An atom can be broken down into 3 constituent parts known as subatomic particles. These parts are neutrons, protons, and electrons. Imagine these particles as tiny spheres in an arrangement that’s similar to that in the image below. It’s worth noting that this is an oversimplification, but it works for the subject we’re discussing.

As we can see in the diagram above, neutrons and protons can be found grouped in the middle of the atom, whilst the electrons can be found orbiting in ‘shells’ around the centre, or the nucleus.
Protons have a positive (+ve) electrical charge, and have a relative atomic mass of 1 (physical measurements of mass are highly impractical at this small scale, so we simply refer to it’s ‘relative mass’). Neutrons also have a relative mass of 1, but carry no electrical charge. Electrons carry a negative (-ve) change, but have negligible mass! Therefore the total ‘mass’ of an atom is the sum of its protons and neutrons only.
A material made up of one type of atom, is known as an element. There are 94 naturally occurring elements. Hydrogen is the lightest, consisting of just 1 proton, 1 electron, and 0 neutrons (so total atomic mass of 1). Uranium is the heaviest, consisting of 92 protons, 92 electrons, and between 141 to 146 neutrons! (Atomic mass of 238). Atoms of the same element with different numbers of neutrons are called isotopes, but we don’t need to go into this for the purpose of this unit.
If we arrange all the elements in order of their number of protons (known as ‘atomic number’), we get a table known as the periodic table.
Electron shells and Valency
So far we have described electrons as orbiting around the central nucleus in ‘shells’. These shells can be thought of as different orbital layers that get ‘filled up; as the atoms go up in size. There are a maximum number of electrons that can fit into any particular shell. Once that shell is full, then another larger shell is formed.
Each of these shells has an energy level, with the shells closest to the nucleus having a lower energy than those further out. If an electron was to move between these shells, it would need an increase in energy to move ‘up’ a shell, or to lose energy to move ‘down’ a shell. Like most things, atoms want to exist in a state requiring the lowest amount of energy, which is why the electrons fill up from the smallest to the largest shells in order.
This diagram shows the first two shells, bear in mind is it also simplified for the purposes of an easier explanation:
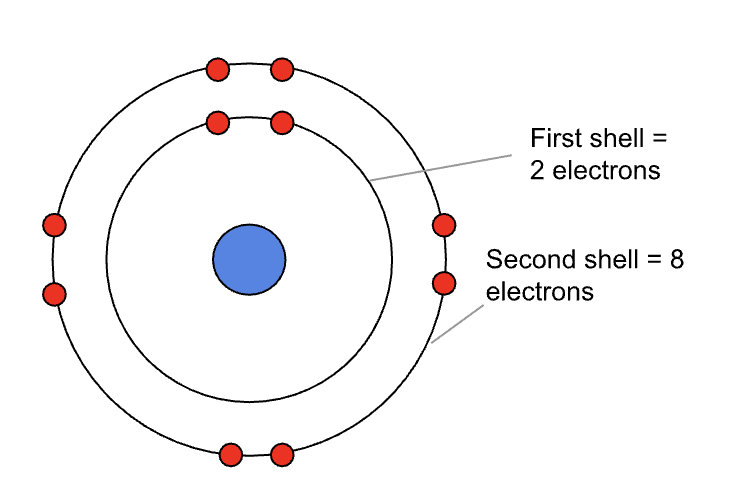
The first shell can hold 2 electrons;
The second shell can hold 8 electrons;
The third shell can hold 18 electrons;
(The general rule for the number of electrons in any shell is 2n2)
Keep an eye out for our next articles looking at the properties of engineering materials.
Interested in our courses?
You can read more about our selection of accredited online mechanical and industrial engineering courses here.
Check out individual courses pages below:
Higher International Diploma in Mechanical Engineering
Higher International Certificate in Mechanical Engineering
Diploma in Mechanical Engineering
Diploma in Mechanical Technology
Higher International Diploma in Industrial Engineering
Higher International Certificate in Industrial Engineering
Diploma in Engineering Management
Alternatively, you can view all our online engineering courses here.
Recent Posts
Kirchhoff’s current and voltage laws
Kirchhoff’s current and voltage laws In our last article, we looked at the principles and operation of a d.c motor. In this article, we’re going to investigate Kirchoff’s current and voltage laws, as well as how to apply them to engineering problems. Kirchoff’s law of current Kirchoff’s law of current states that the algebraic sum […]
What are the principles of operation of a DC electric motor?
What are the principles of operation of a DC electric motor? In our last article, we looked at the electrical parameters in series and parallel electrical circuits. In this article, we’re going to dive into the principles of operation of a DC electric motor. The motor effect When a current-carrying conductor is placed on a […]
What are the electrical parameters in series and parallel electrical networks?
What are the electrical parameters in series and parallel electrical networks? In our last article, we looked at the principles of operation of electrical cells. In this article we’re going to move on to the electrical parameters in both series and parallel electrical networks. When we have circuits with more than one resistor, we need […]

